Abstract
Introduction
Approximately 25-30% of adult acute myeloid leukemias (AMLs) have FLT3 mutation. Among the two main types, internal tandem duplication (ITD) and tyrosine kinase domain (TKD) point mutations, FLT3-ITD is associated with poorer clinical outcomes. Recently, midostaurin was found to improve survival when combined with 7+3 induction for FLT3-ITD or FLT3-TKD AML. Additionally, increased CD33 expression in AML patient samples has been linked to FLT3-ITD+ blasts. This suggests the combined targeting of CD33 and FLT3 in AML patients as a novel treatment approach. Hence, we introduce a combination therapy of gemtuzumab ozogamicin (GO) - a humanized CD33 antibody conjugated to a calicheamicin derivative - and midostaurin with 7+3 (cytarabine + daunorubicin) for newly diagnosed FLT3-mutated AML.
Methods
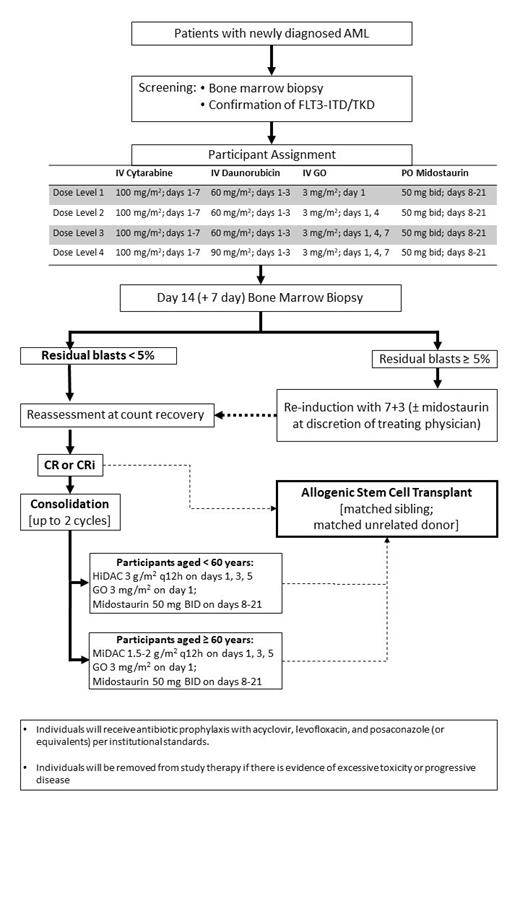
This Phase I open-label, dose-finding study was designed to determine the maximum tolerated dose schedule of GO administered with midostaurin and 7+3 induction and to assess the safety and preliminary efficacy of this combination. Patients needed to be >18 years old, have newly diagnosed AML, be fit for 7+3 induction, and harbor a FLT3-ITD or -TKD mutation per next-generation sequencing or PCR testing. In cohorts of 3, patients were assigned to 1 of 4 dose levels according to the "keyboard" Bayesian toxicity probability interval design (Table 1) applied to a target dose-limiting toxicity (DLT) rate of 20%. Non-hematologic DLTs were defined as study drug-related grade ≥3 toxicities, with exceptions for infections, elevated liver enzymes (that resolve in ≤5 days), GI symptoms (≤3 days), and electrolyte abnormalities (≤1 day). Hematologic DLTs were grade 4 neutropenia or grade ≥3 thrombocytopenia at 6 weeks after the start of the induction cycle and in the absence of AML. The DLT evaluation period covered the induction and, if applicable, re-induction cycles. Clinical responses were determined by 2017 ELN criteria. CD33 expression on AML blasts was quantified before and after treatment. Upon completion of 1 or 2 induction cycles, patients with a CR or CRi could receive up to 2 cycles of consolidation therapy, consisting of cytarabine (HiDAC or MiDAC depending on age <60), midostaurin, and GO on Day 1 of the first cycle, or proceed to allogenic hematopoietic cell transplantation (HCT).
Results
Eight patients have been enrolled out of the planned accrual of 24. The median age was 59 (range: 35-72) years, and every patient identified as White and Non-Hispanic. At screening, all patients had FLT3-ITD mutations. The median bone marrow blast percentage was 64% (range 17%-91%), with the majority of blasts expressing CD33 (median 96%, range 80-100%, 2 patients missing data due to recent enrollment).
The current numbers of patients treated by dose level (DL) were: 3 on DL1 (IV GO 3 mg/m 2 Day 1), 3 on DL2 (IV GO 3 mg/m 2 Day 1 and 4), and 2 on DL3 (IV GO 3 mg/m 2 Day 1, 4, and 7). No DLTs were observed among the six patients in DL1 or DL2. Likewise, no DLTs were observed in the two patients in DL3.
The most common treatment-emergent adverse events included grade 3 febrile neutropenia (75% of patients), grade 3/4 mucositis (25%), grade 3 sepsis (25%), and grade 3 esophagitis (12.5%). One patient had a serious adverse event: a GO-related Grade 4 sinusoidal obstruction syndrome occurring ~4 months into treatment and lasting 23 days. The median duration of study therapy was 62 days (range 20-95 days); reasons for discontinuation were disease progression (n=2), non-compliance (n=1), and allogeneic HCT (n=5).
The overall response rate and composite complete remission rate were both 75% (95% CI: 34.9% - 96.8%). There were no treatment-related deaths in the first 30 days. Due to the early nature of this study, survival outcomes have not been calculated and post-induction CD33 expression data are not available.
Conclusions
In newly diagnosed FLT3-mutated AML patients, induction chemo/immunotherapy with GO, midostaurin, and 7+3 has yielded promising responses and has been well tolerated with no DLTs thus far. Additional patients and correlative analyses of CD33 expression will provide further insight into the safety and efficacy of this regimen for a patient population with historically poor prognosis.
Borate: Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rampal: Membership on an entity's Board of Directors or advisory committees; Galecto, Inc.: Consultancy; Promedior: Consultancy. Saultz: IKENA: Research Funding. Traer: ImmunoGen: Membership on an entity's Board of Directors or advisory committees; Servier/Agios: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Schrodinger: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees. Walter: Amgen: Research Funding; Aptevo: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Immunogen: Research Funding; Macrogenics: Consultancy, Research Funding; Jazz: Research Funding; Pfizer: Consultancy, Research Funding; Selvita: Research Funding; Amphivena: Consultancy, Other: ownership interests; Agios: Consultancy; Astellas: Consultancy; BMS: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Kite: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal